The Logic Behind the Labeling: Answers to Your SPL and SPM Questions
- Marianne Calilhanna

- Aug 5, 2025
- 1 min read
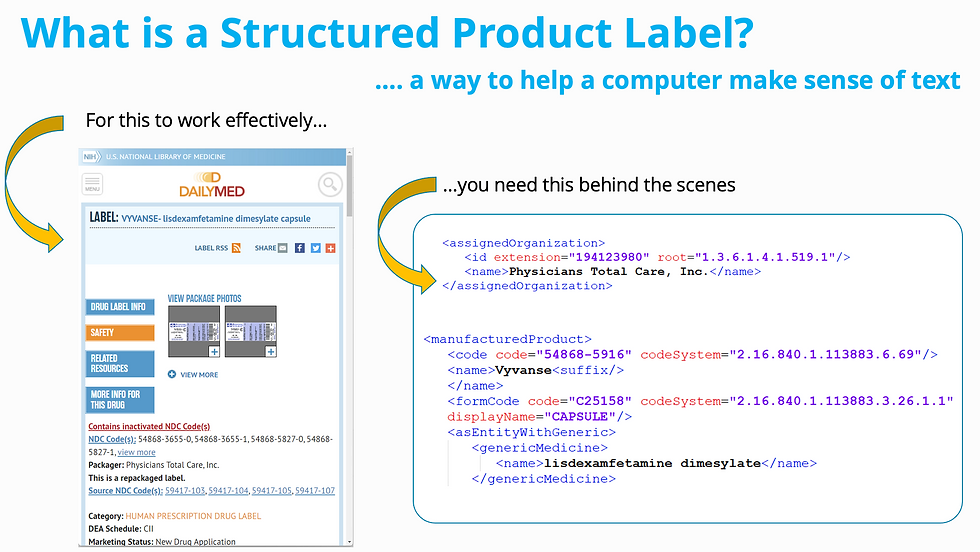
Structured Product Labeling (SPL) and Structured Product Monographs (SPM) play a critical role in how the FDA receives, processes, and evaluates product information. These two acronyms carry a lot of weight in the world of regulatory compliance in the United States. Whether you're just getting started or have experience navigating these structures, DCL provides clarity on key submission requirements, common challenges, and lesser-known nuances that impact compliance and data integrity.



Comments